Fda Health Hazard Evaluation
FDA uses a Health Hazard Evaluation HHE form for classifying recalls as Class I II or III. Ad Get Quote Frictional Force Pinch Test Machine Of Hydrophilic Coatings Catheters.
 Flow Chart From Fda Cdrh Use Of International Standard Iso 10993 Download Scientific Diagram
Flow Chart From Fda Cdrh Use Of International Standard Iso 10993 Download Scientific Diagram
741 Health hazard evaluation and recall classification.

Fda health hazard evaluation. Hazard cannot be assessed with the data currently available. 11102020 The Food and Drug Administration will conduct its own health hazard evaluation and promptly notify the recalling firm of the results of that evaluation if the criteria for recall under. Health Hazard Evaluations HHEs and Health Risk Assessments HRAs are the processes that FDA follows to determine the risks of certain device problems and the actions firms should take to resolve them.
When conducting an HHE companies need review these product complaints in detail. FDAs Health Hazard Evaluation Board has. Ad Search Medical Device Compliance.
Perforation of the intestine. Knowing the likelihood of exposure also is needed for evaluation purposes. From 1972 through 1997 the FDA Health Hazard Evaluation Board evaluated approximately 190 cases of hard or sharp foreign objects in food.
7 mm to 10. See FDA Compliance Policy Guide 555425 FDA 2001a. Glass Inclusion Top Glass fragments can cause injury to the consumer.
The present Health Hazard Evaluation describes the product in question summarizes reports in CAERS of liver-related adverse events associated with ingestion of the product reviews reports of. 272019 Health Hazard Evaluation HHE is a method of classifying recalls and Health Risk Assessment HRA is a method of determining the type and level of risk communication that will be associated with a product or event. They were replaced with the Quality System regulations and included the use of design controls in the development of new medical devices.
Explanation Assess the Probability that Use of or Exposure to Product under Recall will Cause Adverse Health Consequences. FDAs Health Hazard Evaluation Board has supported regulatory action against products with glass 03 inch 7 mm to 1. Get Results from 6 Engines at Once.
Health Hazard Evaluations HHEs and Health Risk Assessments HRAs are the processes that FDA follows to determine the risks of certain device problems and the actions firms should take to. The Federal Food Drug and Cosmetic. Ad Get Quote Frictional Force Pinch Test Machine Of Hydrophilic Coatings Catheters.
10142014 Health hazard analyses HHAs are a necessary part of the medical device manufacturing process since the FDA revised Medical Device Good Manufacturing Practices Regulation 21 CFR Section 820 in 1996. FDAs Health Hazard Evaluation Board has supported regulatory action against products with metal fragments 03 inch 7 mm to 1 inch 25 mm in length. 642015 Your health hazard evaluation is only as good as your complaints management team He added that FDA will always tell companies that the level of its investigation should be directly proportional to the complaint.
Get Results from 6 Engines at Once. 741 Health hazard evaluation and recall classification. A health hazard alone is not enough to assess the risk of the product.
The FDA uses Health Hazard Evaluation HHE to determine a products risk to human health and classify recalls. A An evaluation of the health hazard presented by a product being recalled or considered for recall. 712010 In either of these cases FDA must review the risks to determine the actions needed to resolve them.
Ad Search Medical Device Compliance. A An evaluation of the health hazard presented by a product being recalled or considered for recall will be conducted by an ad hoc committee of Food and Drug Administration scientists and will take into account but need not be limited to the following factors. FDAs Health Hazard Evaluation Board has supported regulatory action against product with metal fragments of 03.
 Approved Risk Evaluation And Mitigation Strategies Rems
Approved Risk Evaluation And Mitigation Strategies Rems
 Risk Assessments Evaluating Foodborne Antimicrobial Resistance In Humans A Scoping Review Sciencedirect
Risk Assessments Evaluating Foodborne Antimicrobial Resistance In Humans A Scoping Review Sciencedirect
 Pdf Human Health Hazard Assessment Of Quaternary Ammonium Compounds Didecyl Dimethyl Ammonium Chloride And Alkyl C12 C16 Dimethyl Benzyl Ammonium Chloride
Pdf Human Health Hazard Assessment Of Quaternary Ammonium Compounds Didecyl Dimethyl Ammonium Chloride And Alkyl C12 C16 Dimethyl Benzyl Ammonium Chloride
 Risk Management Fda S Quality Risk Management Approach To New Drug Applications
Risk Management Fda S Quality Risk Management Approach To New Drug Applications
Biomarkers Fda S Design Control Requirements For Biomarkers In Drug Development
 Pdf Documenting Medical Device Risk Management Through The Risk Traceability Summary
Pdf Documenting Medical Device Risk Management Through The Risk Traceability Summary
Choosing The Right Medical Device Risk Management Tools
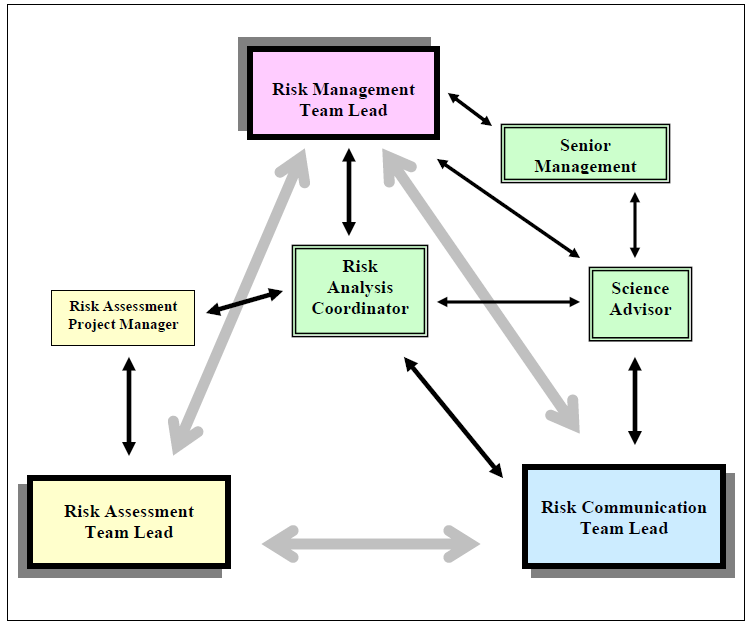
Medical Device Design Risk Management Basic Principles Wipro
Https Www Asiaactual Com Wp Content Uploads 2017 06 Fda Circular No 2016 012 Pdf
Cfr Code Of Federal Regulations Title 21
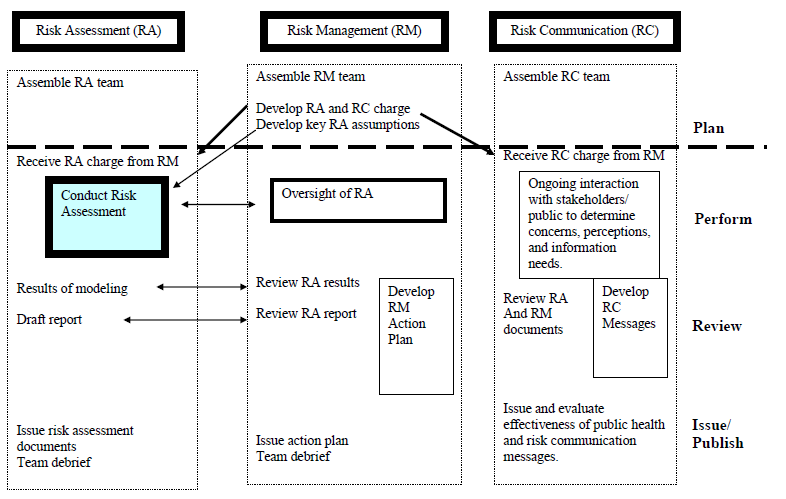
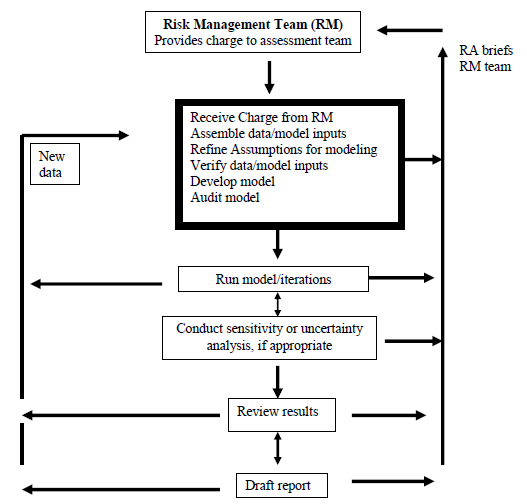
Creating A Medical Device Risk Management Plan And Doing Analysis
 Quality Risk Management Information Training On Risk Management Presentationeze
Quality Risk Management Information Training On Risk Management Presentationeze